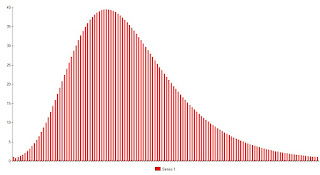
The previous post illustrated the concept of "exposure." It was modeled as an aggregate of encounters that have varying probabilities of transmitting an infection. In the simple case modeled, it was a function of number interactions and the probability of transmitting the disease with each interaction. As mentioned there, these variables are expected to vary from person to person. One person may contract the infection from a low risk encounter, such as using a self serve gas pump after an infected person, or conversing with an asymptomatic infectee from a few feet away. Other people will not become infected despite higher risk exposures. To model this for a population, it is sufficient to create a parameter called "exposure." This is an aggregate of number of exposures, risk per exposure, and individual susceptibility to acquiring infection. In theory, if we could measure and keep track of "exposure" we could derive a detailed epidemiologic model. However, for he sake of illustrating concepts it is sufficient to create an arbitrary exposure profile and illustrate how that affects infection rates. For the sake of illustration, the following figure plots the number of people who acquire an infection against the minimum exposure required to do so. People highly susceptible to infection are on the left hand side of the curve, relatively immune, or those more resistant to infection are found on the right. For example, the first bar on the graph indicates that .004 % of infections for this hypothetical virus occur in people who have the lowest level of exposure, arbitrarily deemed 1 unit; 1.2 percent with an exposure of at least 2 units, etc. The graph is scaled so that the sum of the percentages is 1, representing the entire population that contracts the disease.

Figure 1.
The shape of this particular curve is arbitrary, created merely for illustrative purposes.
Now it would be useful to know how exposures are distributed throughout the at-risk population. To do this, we can plot the percentage of the population that experiences a given exposure. We may get a plot that appears as follows, again created for illustrative purposes only:
Figure 2.
And we can plot them on the same chart:
Figure 3.
The idea to be illustrated here is that the distribution of exposures may differ from the distribution of susceptibility in the population, and this will determine the final percentage of the population that is infected, even if no one had previously been exposed to the disease. Again the key point is that not all exposures have the same probability of passing the infection, and not all people have the same probability of becoming infected from identical exposures. After going through this analysis, we arrive at a common sense conclusion: the fewer exposures that occur in people more resistant to infection, the smaller the percentage of the population that will become infected.
Here is a highly schematic example:
Assume tht we have 300 people. 100 are in the less resistant (more susceptible) group, 200 in the more resistant group. Now let us assume that among these 300 people there are a total of 600 exposures. Each exposure is associated with a 0.5 probability of transmitting infection. Further assume, for purposes of illustration (the purpose is to illustrate the effect of differential distribution, not practical epidemiologic modeling) that these exposures are evenly distributed, so that everyone experiences approximately 2 two exposures. Each person has a 75% chance of being infected (1-(1-.5)^2), so the expected number of infections throughout the population is 300*0.75=225.
Now assume that the exposures are unevenly distributed. Assume that of the 600 exposures 400 occur in the less resistant group and 200 occur in the more resistant group. Again, assume that the exposures are evenly distributed. Now the expected number in the less group is 100*(1-(1-0.5)^4)=94 infections, and in the more resistant group, it is 200*(1-(1-0.5)^1)=100 infections. Now the total number if infections is only 194, rather than 225. This result is due only to redistributing the exposures. It should be noted that, so far "less resistant" and "more resistant" are only labels. Each group, as modeled so far has the same susceptibility sine the risk per exposure is 0.5 in both groups. To justify the labels the probability of infection in the lower resistant group has to be higher than that in the higher resistance group. For the sake of illustration let us keep the probability in the lower resistance group 0.5 but make that in the higher resistance group 0.4. Now the expected number of infections in the higher resistance group is 200*(1-(1-0.4))-80, and the total number if infections is now 174.
We can apply a similar model to the distributions given in Figures 1, 2 and 3. For assumed baseline numbers with a the highest probability of infection per exposure of 0.75, that everyone is equally susceptible and that exposures are evenly distributed, the expected number of infections in a population of 1000 people is 996. For the distributions in Figure 3, assuming a predictable decrease in the probability of infection as we move to the left on the graph, the expected number of infections in the total population is 522. If we swap the profiles, so that the green distribution represents the percent infected as a percentage of risk and the red the distribution represents the distribution of "exposure," the expected number of cases in the population predictably increases to 740.
Again, this just applies numbers to what should be a common sense phenomenon. If we shift the curve of percentage of the population infected as a function of exposure (Figure 1) to the right, and Figure 2 to the left, the total number of infections will go down. In other words, if fewer exposures occur in people who require more exposure to become infected, even if the total number of exposures remains the same, the final number of infections will decrease.
As a practical matter, the red curve is shifted to the right by decreasing the probability of transmission per exposure. The total area of the curve of figure 2 is decreased by minimizing exposures, social distancing, avoiding gatherings, regularly decontaminating surfaces, etc.
A limitation to this type of model, is that merely tries to explain why the total number of people infected during an epidemic is less than 100%, or even the portion arrived at by considering "herd immunity" as the limiting process. What this does not do is account for the expected increase in the amount of the quantity that we simply called "exposure" as the number of cases increases, nor does it give any insight into how an epidemic proceeds over time. That will be addressed in another post.